Nature | Peking University's Deng Hongkui Team Announces a Major Research Breakthrough—Chemical Small Molecules Induce the Transformation of Adult Human Cells into Pluripotent Stem Cells
On April 13, 2022, the research team led by Deng Hongkui from the College of Life Sciences at Peking University and the Peking-Tsinghua Life Sciences Joint Center published a research paper titled "Chemical reprogramming of human somatic cells to pluripotent stem cells" in the international academic journal Nature. For the first time internationally, they reported the breakthrough research achievement of using chemical small molecules to induce the transformation of adult human cells into pluripotent stem cells. The use of chemical small molecules to reprogram cell fate (chemical reprogramming) represents a new generation of self-developed technology for preparing human pluripotent stem cells in China, following "nuclear transfer" and "transcription factor induction." This technology addresses the underlying "bottleneck" issues in the development of stem cells and regenerative medicine in China.
Pluripotent stem cells possess the characteristics of unlimited proliferation and the ability to differentiate into all functional cell types of an organism. These magical traits give them a wide range of application value in fields such as cell therapy, drug screening, and disease modeling, making them the most critical "seed cells" in the field of regenerative medicine. During the natural development process of mammals, pluripotent stem cells only briefly exist in the early stages of embryonic development and then differentiate into various types of adult cells that make up the organism, losing their "seed cell" characteristics. How to reverse this natural development process and make highly differentiated adult cells regain a pluripotent state similar to the early stages of embryonic development has always been one of the most important scientific questions in the field of stem cells and regenerative medicine.
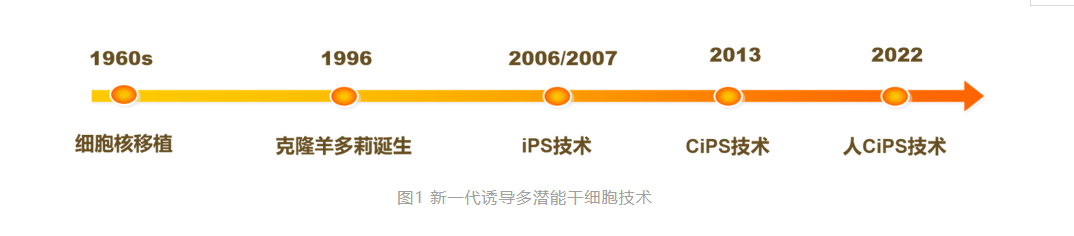
In the 1960s, British scientist John Gurdon developed nuclear transfer technology in frogs, and in 1997, Ian Wilmut's team used this technology to create Dolly the cloned sheep, proving that highly differentiated somatic cells of mammals could also be reversed to the initial state of early embryos and gain the ability to develop into entire animal individuals. In 2006, Japanese scientist Shinya Yamanaka reported that transgenic methods could reprogram mouse somatic cells into pluripotent stem cells, known as induced pluripotent stem cells (iPS cells). Nuclear transfer and the introduction of foreign genes have proven that mammalian somatic cells can be reprogrammed to reverse to the early stages of embryonic development and regain "pluripotency." These two technologies were awarded the Nobel Prize in Physiology or Medicine in 2012. The establishment of iPS technology broke through the ethical limitations of traditional embryonic stem cells and provided a brand-new method for constructing patient-specific stem cell lines, greatly accelerating the process of stem cell clinical applications. In recent years, cell therapy clinical trials have been conducted for a variety of major diseases, including Parkinson's, diabetes, and cancer. However, the current cell therapy technical systems have all been developed abroad. Can China have original underlying technology?
For a long time, Deng Hongkui's team has been committed to developing new methods to control cell fate and establishing underlying technologies for preparing stem cells. In 2013, Deng Hongkui's team published an original research result in Science magazine, that is, without relying on oocytes and transcription factors and other endogenous cellular materials, cells can be reprogrammed to reverse their fate, turning mouse somatic cells into pluripotent stem cells (chemically induced pluripotent stem cells, CiPS cells) using only exogenous chemical small molecules. Compared with traditional methods, chemical small molecules are easy to operate and flexible, with strong spatial and temporal control, reversible effects, and can accurately control the cell reprogramming process. In addition, the non-integrative method of small molecule-induced somatic cell reprogramming avoids safety issues caused by traditional transgenic operations and is expected to become a safer clinical treatment method. Subsequently, Deng Hongkui's team published articles in Cell and Cell Stem Cell magazines, detailing the unique molecular mechanisms of chemical reprogramming and further optimizing the mouse chemical reprogramming system. Subsequently, including Xie Xin, Yao Hongjie, Pei Duanqing, Liu Lin, Zhu Saiyong, and other research groups used the same or similar combinations of chemical small molecules to replicate and optimize the mouse chemical reprogramming technology. Chemical reprogramming to induce pluripotent stem cells has opened up a brand-new somatic cell reprogramming pathway, which not only helps to better understand the mechanisms of cell fate determination and transformation but also brings new possibilities for future regenerative medicine treatment of major diseases.
In this study, Deng Hongkui's team reported for the first time the use of chemical reprogramming methods to successfully induce adult human cells into pluripotent stem cells (human CiPS cells). The establishment of this technology has opened up a brand-new path for the preparation of human pluripotent stem cells, taking a key step towards clinical application.
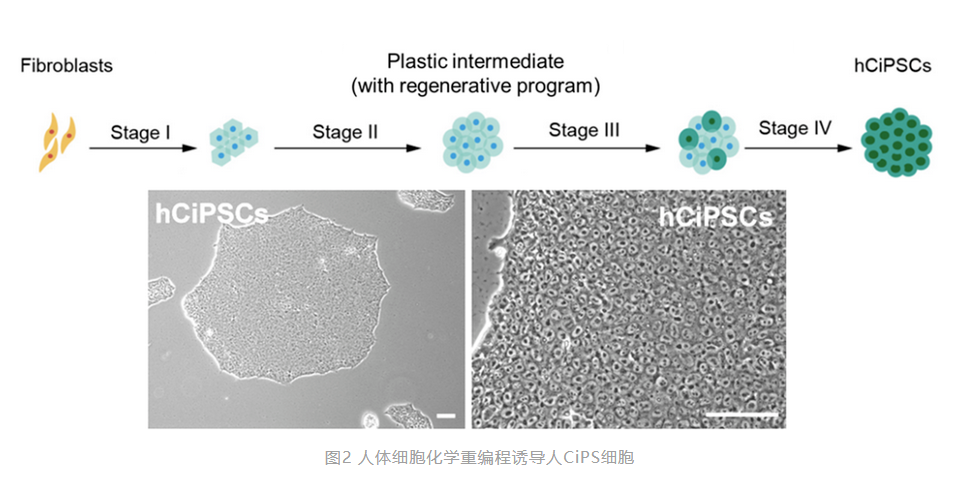
As a higher animal, the complexity of human adult cell characteristics and homeostasis regulation is far from comparable to that of mouse adult cells. There are many obstacles at the epigenetic level, which severely limit the possibility of stimulating cellular plasticity in human adult cells. Since 2013, although many international teams have made numerous attempts inspired by the work of mouse chemical reprogramming, they have not been able to solve the problem of chemical reprogramming of human adult cells. This has led the field to generally believe that the epigenetic restrictions of human adult cells are extremely strict and may not be able to stimulate human adult cells to obtain pluripotency through chemical reprogramming. Deng Hongkui's team, after a long-term persistence and unremitting efforts, has broken through this bottleneck. The key step of this breakthrough was inspired by the regeneration process of lower animals. Newts and other lower animals, after being damaged by the external environment, will spontaneously change their own characteristics, then through dedifferentiation, obtain a certain plasticity, and rely on this plastic intermediate state to achieve limb regeneration. Following this idea, the research team conducted a large number of screenings and combinations of chemical small molecules, and finally found that highly differentiated human adult cells can also undergo a similar dedifferentiation phenomenon under the action of a specific combination of chemical small molecules, obtaining an intermediate state with a certain plasticity. On this basis, the research team finally achieved the successful induction of human CiPS cells.
Compared with traditional technology systems, CiPS cell induction technology has irreplaceable advantages such as greater safety and simplicity, ease of standardization, and controllability, breaking through the limitations of iPS technology, and has a broad prospect for clinical application. 1) In terms of safety, it has been proven in mouse CiPS cells that they carry significantly fewer genetic mutations than traditional iPS cells, and the chimeric mice did not develop tumors and all survived healthily during a six-month observation period. At the same time, islet cells derived from human CiPS cells transplanted into mice and non-human primate models showed no tumor formation after long-term observation; 2) In terms of individualized preparation, the research team has now been able to stably induce human CiPS cells from somatic cells of different ages; 3) In terms of cell standardization, chemical small molecules have a series of characteristics such as simple operation, strong spatial and temporal control, reversible effects, convenient synthesis and storage, and easy standardization production, making human CiPS cells have irreplaceable advantages in standardization and large-scale production. It is particularly worth noting that human CiPS cells can efficiently prepare islet cells, and safely and effectively improve the blood sugar control of diabetic monkeys, highlighting the huge advantages of human CiPS cells as "seed cells" for treating major diseases in terms of safety and effectiveness. The main results of this study were published in the medical journal Nature Medicine in February this year.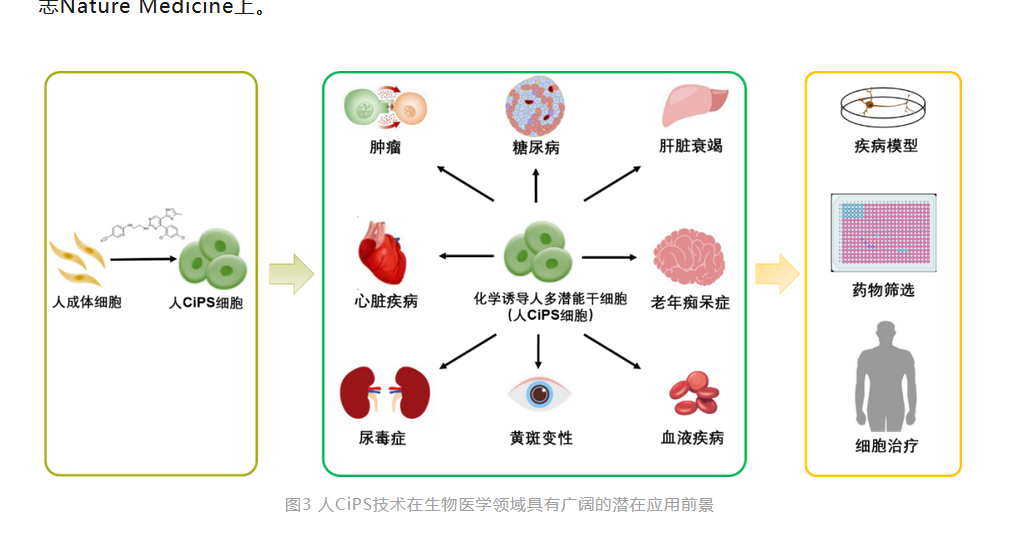
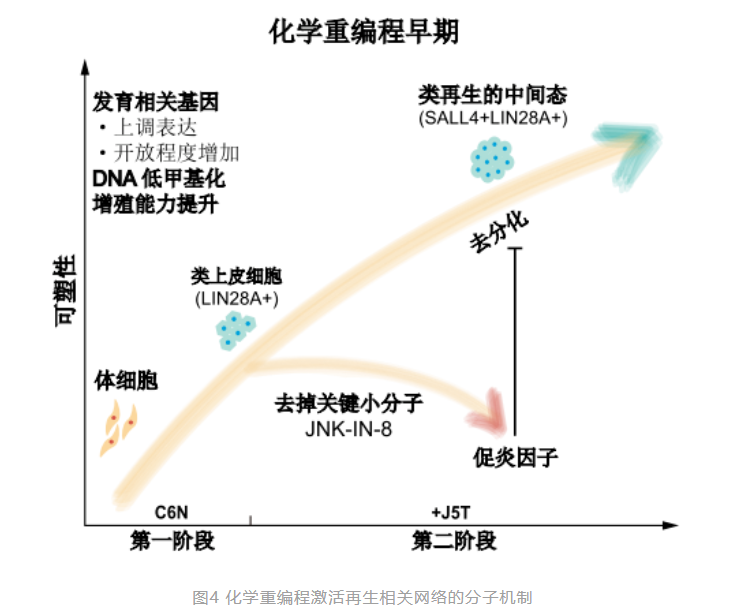
Based on this, the research team also depicted the molecular pathway of chemical reprogramming inducing human CiPS cells, revealing the molecular mechanism and unique regulatory mechanism of chemical reprogramming different from traditional transcription factor reprogramming. The study found that the induction of human CiPS is a staged and precise regulation process, producing a special intermediate state in the early stage. By comparing the cell properties in detail with the regeneration dedifferentiation process of lower animals, the research team confirmed that the early stage of human CiPS cell induction activates gene expression characteristics similar to those in the early stages of limb regeneration in lower animals. More importantly, the research team also discovered the key signaling pathways that regulate this type of regenerative state, proving that inhibiting excessive inflammatory responses is crucial for chemical reprogramming to induce human cells to regain a regenerative intermediate state. This regenerative intermediate state provides a new idea for studying the reactivation of regeneration genes in human cells and suggests the possibility of reactivating the plasticity and regenerative potential of human cells by only using a combination of chemical small molecules in the future, which is expected to promote the application of chemical reprogramming in tissue and organ regeneration, providing new possible pathways for regenerative medicine research.
In recent years, Deng Hongkui's team has expanded the strategy of chemical reprogramming to various aspects of controlling cell fate. The team has used chemical small molecules to achieve the transformation between different somatic cell types, directly reprogramming skin cells into functional neurons (Cell Stem Cell, 2017)This series of work demonstrates the universality of chemical small molecules in regulating cell fate.
In summary, the establishment of the chemical reprogramming technology system not only holds great significance and value in the clinical application field of pluripotent stem cells but also provides a new perspective and platform for the theoretical study of cell fate control and regenerative biology. Chemical reprogramming can precisely control cell fate and is expected to become a versatile technology for efficiently preparing various types of functional cells, paving new ways to treat major diseases.